
PLATELET-RICH PLASMA AS A SAFE AND EFFECTIVE AUTOLOGOUS TREATMENT FOR CHRONIC WOUNDS
Solange Vischer, Senior Scientific Advisor | Regen Lab SA | Switzerland – Antoine Turzi, CEO | Regen Lab SA | SwitzerlandPlatelet-rich plasma (PRP) is an autologous biologic drug prepared from the patient’s blood and used as a treatment for wound healing and other lesions on the same patient.
It is still considered by many to be an experimental treatment and is not covered by insurance even though there is now a significant medical consensus among clinicians around the world who have published studies on the safety and effectiveness of using PRP for chronic wounds.
The United States became a pioneer in this field by deciding in 2021 to take over the coverage of autologous PRP gel treatments for the treatment of non-healing diabetic chronic foot wounds.
Various recent meta-analyses, summarized here, have compiled and synthesized clinical studies done on patients with chronic wounds and treated with PRP gels compared to standard treatments. These meta-analyses demonstrate the safety and efficacy of PRP gels for the treatment of chronic wounds such as diabetic foot ulcers, venous leg ulcers, and pressure ulcers.
Keywords: Chronic wounds, diabetic foot ulcer, venous ulcer, pressure ulcers, platelet-rich plasma, PRP, medical devices, regulation, MDR 2017/745, FDA, MDSAP, ISO13485
INTRODUCTION
In April 2021 in the United States, the Centers for Medicare & Medicaid Services released the Determination of National Coverage (NCD 270.3) for the management of autologous PRP for the treatment of chronic non-healing diabetic wounds under Section 1862(a)(1)(A) of the Social Security Act.
A treatment duration of 20 weeks is covered by insurances when prepared by devices, such as RegenKit® Wound Gel-2, whose FDA-approved indications include the management of exuding skin wounds, such as diabetic ulcers.
Regen Lab, Switzerland has been certified ISO13485 since 2003 for the manufacture and marketing of RegenKits internationally. In Europe, RegenKits have already obtained their CE certificate according to MDR 2017/745. In the United States, RegenLab® USA received its first FDA clearance in May 2010 with RegenKit® THT®, which has been part of a family of medical devices manufactured in the United States since November 2021.
In accordance with the MDSAP ISO-13485 standard, the production of RegenKits in Europe and the USA follows the highest quality management standards.
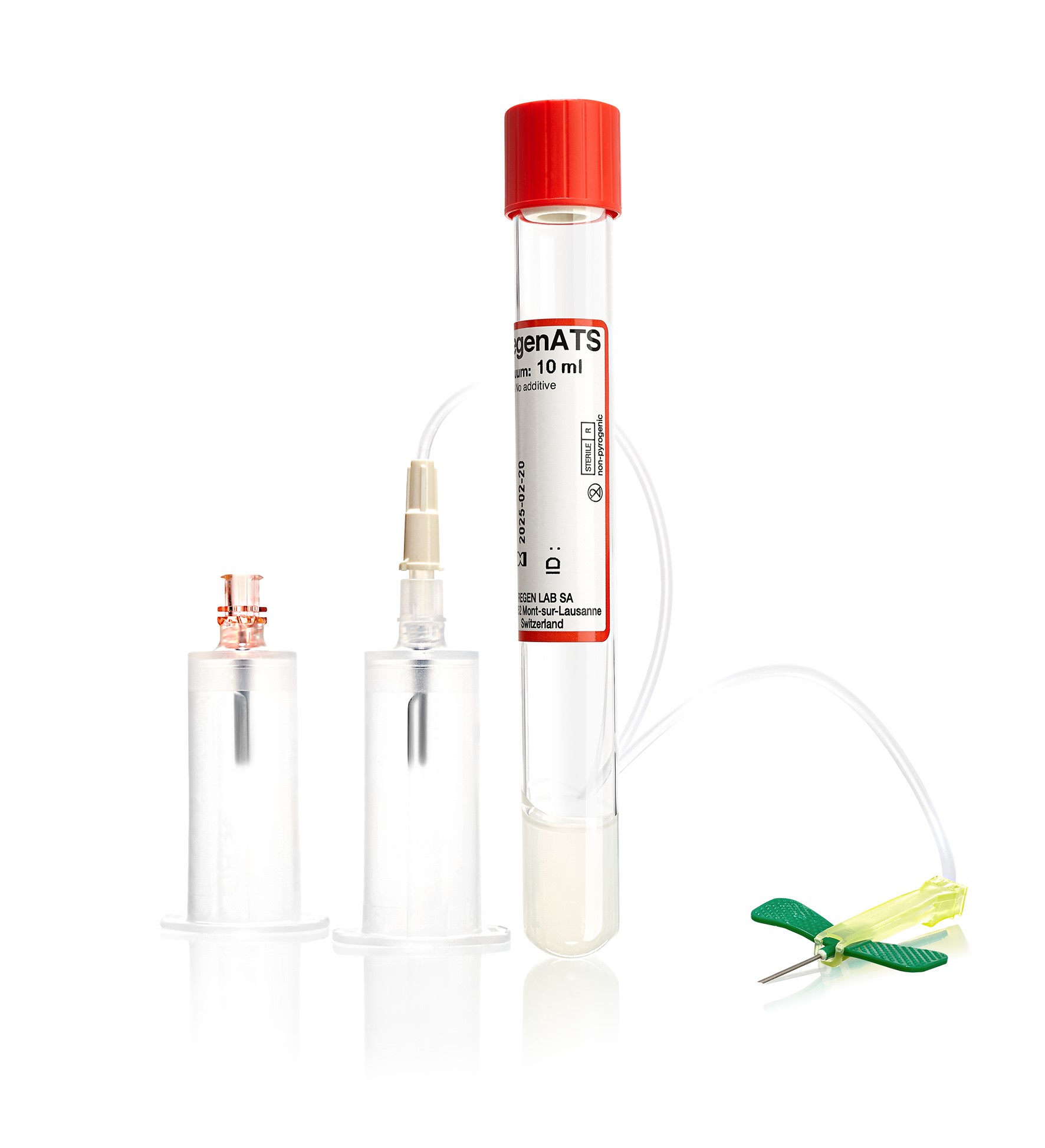
The RegenKit® technology enables a fast and standardized PRP preparation process with a closed-circuit system. This PRP (RegenPRP) has demonstrated its safety, reliability and efficacy in more than 300 scientific and clinical publications in academic journals.
Autologous PRP is a suspension of platelets in plasma, prepared from the patient’s blood and used as a treatment for wound healing and other lesions on the same patient, thus minimizing the possibility of crossreactivity and allergic reactions. PRP contains live, functional platelets, so this biological preparation is different from other autologous blood-derived growth factor preparations such as clot extracts.
Similarly, frozen/thawed PRP should not be considered equivalent to fresh PRP as most platelets do not survive this process [1].
RegenKit® technologies, such as RegenKit® Wound Gel-2, produce PRP treatments from fresh, minimally manipulated blood to harness and maximize the patient’s natural healing capacity. Devices used for the isolation of PRP from the patient’s blood relate to the definition of a medical device and must therefore comply with the regulations in force.
Over the past 20 years, multiple technologies for the preparation of PRP at the bedside of the patient have been approved by the health authorities of each country.
In the European Union, they are governed by Regulation 2017/745 (MDR), which has replaced Directive 93/42/EEC (MDD) since 2021.
In the United States, devices for the preparation of a PRP gel or membrane used as a biological dressing for wound care are regulated by the CBER (Center for Biologics Evaluation and Research) of the Food and Drug Administration (FDA). They carry the product code PMQ and follow section 864.9246 of the Code of Federal Regulation Title 21 (21 CFR 864.9245) for automated blood cell separators.
The FDA-approved devices for wound care are:
• Autologel System, Cytomedix, Inc, USA, (BK060007)
• 3C Patch System, Reapplix Aps, Denmark, (BK140211, BK170002, BK200471)
• RegenKit® Wound Gel-2, Regen Lab SA, Switzerland, (BK210661)
• Another family of devices has been approved with the PMQ product code, but these devices (RD1 and RD2 Systems, RedDress Ltd, Israel, (BK170095, BK190349, BK200464, BK210570)) are not used to prepare a PRP gel but a whole blood clot combined with kaolin powder.
Compared to other devices designed to prepare PRP, RegenLab® devices produce PRP with a standardized composition.
The use of thixotropic separator gels with specific densities allows for precise isolation at the cellular level of the PRP of other blood components. This method of fractionating blood is very reproducible since it is independent of the operator and the patient. The resulting PRP, RegenPRP, is a low-leukocyte PRP in which there is a specific depletion of pro-inflammatory neutrophil granulocytes. The platelet recovery rate in RegenPRP is greater than 80% with no specific loss of the largest and densest platelets that are known to be the richest in growth factors [2].
This standardized PRP has been shown to be effective in many different therapeutic areas. Regarding chronic wounds, RegenPRP has been used successfully in ten studies with a total of 213 patients [3, 4, 5, 6, 7, 8, 9, 10, 11, 12].
All these studies have shown that the use of RegenPRP allows patients to achieve reepithelialization and closure of chronic wounds such as diabetic foot ulcers, venous ulcers, and other chronic wounds of various aetiology.
Specifically, with respect to diabetic foot ulcers, platelet gels prepared with RegenPRP have demonstrated their safety and efficacy in clinical evaluations.
- RegenWound Gel (RWG) therapy was studied in a single-centre, open-label, randomized, concurrent controlled trial, where ninety-one subjects were evaluated (47 in the RWG group, 44 in the control group). Enrolled patients were diagnosed with type 1 or type 2 diabetes, with one or more diabetic foot ulcers (DFUs), classified as grade 3A according to the University of Texas All subjects received standard treatment (TS), including wound cleaning, removal of necrotic or infected tissue, management of wound infection, treatment of bone complications, and appropriate off-loading. In addition to the TS, the RWG group received an application of RegenPRP gel every 2 to 3 weeks when deemed necessary for a maximum of 6 weeks. At week 6, 55.3% of subjects in the RWG group had their ulcer closed, while this value was 25.6% for the control group. At week 12 (i.e., at the end of the study visit), the ulcer closure rate was 77.3% in the RWG group compared to 35.1% in the control group [13].
- Another clinical study that evaluated the safety and efficacy of RegenPRP autologous gel for UPDs examined the treatment of patients with concomitant peripheral arterial disease (PAD) and diabetic foot ulcers, which are classified as grade 1C according to the University of Texas classification. Thirty of the seventy-two subjects studied suffer from severe PAD, classified as critical limb ischemia. By treating all patients with Autologous RegenPRP Gel, 100% of the limbs were saved in the 42 patients who had DPUs with non-critical PAD, and 73% of the limbs were saved with the 30 patients who had DPUs with critical PADs, for an average of 89% limb recovery [9].
- Russo et al. performed a study to determine the cost-effectiveness of RegenPRP gel treatment compared to standard treatment from the perspective of the French healthcare system [14]. A cost-effectiveness analysis was performed using a Markov decision model in a cohort of patients with chronic UFD (duration of >3 weeks) at high orthopaedic risk and with ulcers classified as 3A according to the University of Texas classification. Efficacy results were reported in terms of quality- adjusted life year (QALY). Costs were reported in euros (€) assessed in 2019. A micro-cost approach alongside a clinical study was used to assess resource utilization. The incremental cost-effectiveness ratio of PRP treatment was €–613/QALY, which, being less than zero, indicates the predominance of PRP They concluded that RegenPRP gel was a cost-effective alternative to standard care due to improved complete cure rates.
- RegenWound Gel (RWG) therapy was studied in a single-centre, open-label, randomized, concurrent controlled trial, where ninety-one subjects were evaluated (47 in the RWG group, 44 in the control group). Enrolled patients were diagnosed with type 1 or type 2 diabetes, with one or more diabetic foot ulcers (DFUs), classified as grade 3A according to the University of Texas All subjects received standard treatment (TS), including wound cleaning, removal of necrotic or infected tissue, management of wound infection, treatment of bone complications, and appropriate off-loading. In addition to the TS, the RWG group received an application of RegenPRP gel every 2 to 3 weeks when deemed necessary for a maximum of 6 weeks. At week 6, 55.3% of subjects in the RWG group had their ulcer closed, while this value was 25.6% for the control group. At week 12 (i.e., at the end of the study visit), the ulcer closure rate was 77.3% in the RWG group compared to 35.1% in the control group [13].
- Another clinical study that evaluated the safety and efficacy of RegenPRP autologous gel for UPDs examined the treatment of patients with concomitant peripheral arterial disease (PAD) and diabetic foot ulcers, which are classified as grade 1C according to the University of Texas classification. Thirty of the seventy-two subjects studied suffer from severe PAD, classified as critical limb ischemia. By treating all patients with Autologous RegenPRP Gel, 100% of the limbs were saved in the 42 patients who had DPUs with non-critical PAD, and 73% of the limbs were saved with the 30 patients who had DPUs with critical PADs, for an average of 89% limb recovery [9].
- Russo et al. performed a study to determine the cost-effectiveness of RegenPRP gel treatment compared to standard treatment from the perspective of the French healthcare system [14]. A cost-effectiveness analysis was performed using a Markov decision model in a cohort of patients with chronic UFD (duration of >3 weeks) at high orthopaedic risk and with ulcers classified as 3A according to the University of Texas classification. Efficacy results were reported in terms of quality- adjusted life year (QALY). Costs were reported in euros (€) assessed in 2019. A micro-cost approach alongside a clinical study was used to assess resource utilization. The incremental cost-effectiveness ratio of PRP treatment was €–613/QALY, which, being less than zero, indicates the predominance of PRP They concluded that RegenPRP gel was a cost-effective alternative to standard care due to improved complete cure rates.
RECENT META-ANALYSES ON THE USE OF AUTOLOGOUS PRP FOR CHRONIC WOUNDS
Chronic Wounds
Li et al. (2023) conducted a study to compare PRP gels with normal saline dressings in the treatment of chronic wounds [15]. Three hundred and thirty (330) patients with chronic wounds, reported in eight randomized controlled studies (RCTs), were included in this study. A total of 169 out of 330 (51.21%) were treated with PRP gels and 161 out of 330 (48.79%) were treated with normal saline dressings. The pooled results showed that the complete cure rate of the PRP group was significantly higher than that of the saline group at 8 weeks and 12 weeks, respectively. In addition, there were no significant differences in wound infection rates and adverse events between the two groups. Compared with normal saline dressing, PRP gel could effectively improve the prognosis of chronic wounds. In addition, PRP gels have been shown to be safe as they have not increased the rate of wound infection or the occurrence of adverse events. They concluded that PRP should be available for the treatment of chronic wounds.
Meznerics et al. (2022) investigated the therapeutic effects of platelet-rich plasma on the treatment of chronic wounds [16]. They identified 48 eligible RCTs comparing PRP with conventional ulcer treatment. Thirty-three study groups of 29 RCTs with a total of 2198 wounds showed that the chances of complete closure were significantly higher in the PRP group than in the control group (OR = 5.32; CI: 3.37; 8.40; I2 = 58%). They concluded that PRP is a safe and effective modality for improving wound healing. By implementing it in clinical practice, platelet-rich plasma could become a valuable and widely used tool, as it could not only improve patients’ quality of life but also reduce the healthcare burden related to wound management.
Diabetic foot ulcers
Peng et al. (2024) conducted a systematic review of RCTs comparing autologous PRP with conventional treatments for diabetic foot ulcers, according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [17].
A total of 10 RCTs involving 550 patients (279 PRP and 271 conventionally treated) were included. In this study, PRP was found to significantly improve the cure rate (risk ratio [RR] = 1.38, 95% confidence interval [CI] 1.05 – 1.82, p = 0.02) and shorten the cure time (mean difference [MD] = -23.23, 95% CI
-45.97 to -0.49, p = 0.05) of patients with UFD compared to conventional treatment.
The available data suggest that the incidence of adverse events was lower in the PRP group than in the conventional treatment group.
The authors concluded that, compared to conventional treatment, PRP effectively promoted recovery in patients with UFD by undoubtedly improving the cure rate and healing time.
Platini et al. (2024) conducted a systematic review of randomized controlled trials to evaluate the safety and efficacy of autologous PRP gel as a novel treatment for diabetic foot ulcers compared to standard treatment in adult patients. Eight RCTs involving 598 patients were eligible for this analysis.
They concluded that, compared to standard care/conventional treatment, autologous PRP gel could significantly improve the cure rate, shorten the healing time, shorten the length of hospital stay, and reduce the amputation rate [18].
Ruiz-Munoz et al. (2024) conducted a systematic review of randomised controlled trials examining the effect of PRP compared to conventional treatments on the rate of ulcer healing in diabetic patients. Results were reported according to PRISMA guidelines.
Eleven articles were included in this review. The studies included a total of 418 individuals in the experimental group and 410 individuals in the control group, resulting in a total of 828 participants. The quality of the studies was assessed using the CASPe tool for critical reading of scientific evidence in the 11 clinical trials included in this review. All studies passed the assessment, with scores ranging from 10 to 11 out of a total of 11 points, indicating high quality.
They found that PRP treatment significantly increases the rate of ulcer healing compared to existing conventional treatments and concluded that PRP can be considered the first choice for treating ulcer closure and healing in diabetic patients [19].
Deng et al. (2023) conducted a systematic review and meta-analysis of randomized controlled trials to review, evaluate, and synthesize the scientific evidence on the safety and therapeutic efficacy of autologous PRP in the management of diabetic foot ulcers compared to conventional or alternative therapy [20]. A total of 22 articles were included. The selected trials involved a total of 1559 people who had diabetic foot ulcers. Of these participants, 785 were treated with PRP, while the remaining 774 were assigned to a control group. The results of the meta-analysis indicate that autologous PRP has a significant positive effect on the healing rate (RR = 1.42, 95% CI 1.30-1.56, p < 0.001), reduces healing time (MD =
. 3.13, 95% CI . 5.86 to . 0.39, p < 0.001), accelerates ulcer area reduction (MD = 1.02, 95% CI 0.51- 1.53, p<0.001), decreased the rate of amputation (RR = 0.35, 95% CI 0.15-0.83, p<0.001) and did not increase the incidence of adverse events (RR = 0.96, 95% CI 0.57-1.61, p>0.05) compared to conventional treatment.
The results of this systematic review and meta-analysis indicate that the use of autologous PRP therapy
is a viable and safe therapeutic approach for diabetic foot ulcers, as it effectively improves wound healing.
Gong et al. (2023) performed a meta-analysis to assess the effect of platelet-rich plasma compared to standard management for the treatment of diabetic foot ulcers (21). A systematic literature search, up to March 2022, was conducted on 19 studies including 1435 subjects with diabetic foot ulcer wounds at baseline; 723 of them were treated with platelet-rich plasma and 712 received standard care. They found that the use of autologous platelet-rich plasma resulted in significantly higher healing of diabetic foot ulcers compared to control treatment (P < 0.001).
OuYang et al. (2023) evaluated the efficacy of PRP therapy for the treatment of diabetic foot ulcers in 20 controlled studies according to PRISMA guidelines [22]. These studies included 1266 patients, of whom 698 were treated with PRP. The authors found that PRP significantly improves the cure rate (p < 0.001) and shortens the healing time (p < 0.001) of patients with UFD compared to conventional treatment.
Su et al. (2023) investigated the efficacy and safety of autologous PRP for the treatment of diabetic foot ulcers [23] Seventeen studies with a total of 1303 participants (649 randomised to the PRP group and 654 to a standard care group) met the eligibility criteria and were included in the study. Compared to standard care, PRP appeared to promote the rate of complete cure (odds ratio (OR): 2.11; 95% confidence interval: 1.55-2.86). PRP also appears to significantly shorten the time to complete recovery (mean duration: -19.04 days; 95% CI: -20.46–17.61). There was no significant difference in adverse events. The results of the sensitivity analyses were robust. They concluded that PRP is an effective and safe adjuvant treatment for diabetic foot ulcers.
Venous leg ulcers
Hu et al. (2024) evaluated the efficacy and safety of PRP compared to conventional therapy for the treatment of venous ulcers. A systematic review of four databases identified 16 randomised clinical trials involving 699 patients. PRP significantly improved the complete healing of the ulcer and increased the percentage of the healed ulcer area by an average difference of 47%. In addition, PRP
shortened the time to complete recovery by an average of 3.25 months. PRP significantly decreased ulcer recurrence without increasing the risk of infection or irritative dermatitis.
They concluded that PRP is a viable and safe alternative for the treatment of venous ulcer, bringing significant improvements in healing outcomes and that integrating
PRP into standard clinical procedures has the potential to improve patients’ quality of life and reduce healthcare burden [24].
Fang et al. (2023) evaluated the clinical effects of platelet-rich plasma in the treatment of venous lower limb ulcers using a meta-analysis method [25]. A total of 294 patients with venous lower limb ulcers from 6 studies were included in the meta-analysis. There were 148 patients in the experimental group treated with PRP compared to 146 patients in the control group treated with conventional therapy. The difference between the cure rate of the experimental group and that of the control group was statistically significant.
This study suggested that the application of PRP for venous ulcers of the lower extremities accelerates the wound healing process and improves wound healing rates.
Yammine et al. (2022) conducted a systematic review to assess the effectiveness of PRP compared to the standard of care commonly used to treat venous lower limb ulcers (26). Ten prospective studies (8 randomised) met the inclusion criteria and included 451 patients with 527 venous ulcers. The results were as follows: a) the weighted odds ratio of the mean cure rate was 2.84 (95% CI = 1.160 to 5.056, I2 = 41.4%, p = 0.0004), b) the mean areas of healed ulcers were 79.2 ± 19% for the PRP group and
51.7 ± 36% for the control group (p = 0.007) in favour of the PRP group, and (c) the weighted odds ratio of the infection rate showed no significant difference between the two groups.
In addition, negative correlations were found between the healing rate and duration of venous ulcers and the initial size of the ulcers. This meta-analysis demonstrated significant beneficial effects of autologous PRP compared to standard care on the cure rate, surface area reduction and reduction of the healing time of venous ulcers. Infection and other complications were similar to standard care. They concluded that their analytical data support the use of PRP as a safe and effective treatment for venous ulcers.
Bedsores
Hu et al. (2024) explored the potential of GWP for pressure ulcers (27). Their meta-analysis of 9 RCTs, involving 511 patients with 523 pressure ulcers, revealed a significant improvement in the cure rate (p < 0.000l). In addition, the standard mean difference for the area of the healed ulcer favoured the PRP group, reflecting an improvement of 1.38 cm2 (p = 0.02).
The reduction in pressure ulcer healing assessment scores in the PRP group surpassed that observed
in the control group, demonstrating a standard mean difference of 1.69 (p = 0.01).
They concluded that PRP stands out as a promising and safe therapeutic approach for pressure ulcers.
CONCLUSION
Numerous clinical studies, systematic reviews of the literature, and meta-analyses have demonstrated the value of PRP in the treatment of chronic wounds, including diabetic foot ulcers, venous leg ulcers, and pressure ulcers.
Recent publications confirm the trend observed in previous meta-analyses and reviews which have all concluded that PRP improves the healing rate of chronic wounds and does not raise any safety concerns [28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38].
Chronic wounds represent a silent epidemic that affects a large part of the world’s population, especially
vulnerable groups such as the elderly and the socio-economically disadvantaged.
In developed countries, it is estimated that 1 to 2 percent of the population will suffer from a chronic wound in their lifetime [39].
The dramatic increase in population aging will only increase these numbers. Platelet-rich plasma, when prepared from the patient’s own blood with medical devices approved by the authorities, has proven to be a safe and effective treatment with significant therapeutic and medico-economic benefits for the management of exuding skin wounds, such as diabetic ulcers and venous leg ulcers.
The standardized RegenKit® Wound Gel technology is indicated for the treatment of chronic wounds and facilitates the efficient and reproducible production of an autologous PRP gel at the point of care, ensuring the highest level of quality and safety for the delivery of these effective and cost-effective treatments to patients suffering from chronic wounds.
REFERENCES
1. Perut F, Filardo G, Mariani E, Cenacchi A, Pratelli L, Devescovi V, et al. Preparation method and growth factor content of platelet concentrate influence the osteogenic differentiation of bone marrow stromal cells. Cytotherapy 2013; 15(7): 830-9. http://www.ncbi. nlm.nih.gov/pubmed/23731763
2. Corash L, Tan H, Gralnick HR. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood 1977; 49(1): 71-87. http://www. ncbi.nlm.nih.gov/pubmed/830377
3. Cervelli V, De Angelis B, Lucarini L, Spallone D, Balzani A, Palla L, et al. Tissue regeneration in loss of substance on the lower limbs through use of platelet-rich plasma, stem cells from adipose tissue, and hyaluronic acid. Adv Skin Wound Care 2010; 23(6): 262-72. https:// www.ncbi.nlm.nih.gov/pubmed/20489388
4. Cervelli V, Lucarini L, Spallone D, Brinci L, de Angelis B. Use of platelet rich plasma and hyaluronic acid on exposed tendons of the foot and ankle. J Wound Care 2010; 19(5): 186, 8-90. https://www.ncbi.nlm. nih.gov/pubmed/20505591
5. Cervelli V, Lucarini L, Spallone D, Palla L, Colicchia GM, Gentile P, et al. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Adv Skin Wound Care 2011; 24(4): 176-81. https://www.ncbi.nlm.nih.gov/pubmed/21422842
6. Cervelli V, Gentile P, Brinci L, Pasquali CD, Bocchini I, Angelis BD. Use of Platelet Rich Plasma (PRP) and Hyaluronic Acid in Treatment of Extremity Gunshot Injuries: A Case Report. World journal of plastic surgery 2016; 5(1): 80-4. https://www.ncbi.nlm.nih.gov/ pubmed/27308248
7. Filomia D, Ventura C, Crescibene A, Almolla J, Napolitano M. Treatment
of pilonidal sinus disease with autologous platelet-rich plasma. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia 2013; 148(6): 704-6. https://www.ncbi.nlm.nih.gov/pubmed/24442056
8. Gentile P, De Angelis B, Agovino A, Orlandi F, Migner A, Di Pasquali C, et al. Use of Platelet Rich Plasma and Hyaluronic Acid in the Treatment of Complications of Achilles Tendon Reconstruction. World journal of plastic surgery 2016; 5(2): 124-32. https://www.ncbi.nlm.nih.gov/pubmed/27579267
9. Kontopodis N, Tavlas E, Papadopoulos G, Pantidis D, Kafetzakis A, Chalkiadakis G, et al. Effectiveness of Platelet-Rich Plasma to Enhance Healing of Diabetic Foot Ulcers in Patients With Concomitant Peripheral Arterial Disease and Critical Limb Ischemia. The international journal of lower extremity wounds 2016; 15(1): 45-51. https://www.ncbi.nlm.nih.gov/ pubmed/25795280
10. Monami M, Mirabella C, Scatena A, Nreu B, Zannoni S, Aleffi S, et al. CO(2) laser for the treatment of diabetic foot ulcers with exposed bone. A consecutive series of type 2 diabetic patients. Journal of endocrinological investigation 2017; 40(8): 819-22. https://www.ncbi.nlm.nih.gov/pubmed/28260184
11. Rainys D, Cepas A, Dambrauskaite K, Nedzelskiene I, Rimdeika R. Effectiveness of autologous platelet-rich plasma gel in the treatment of hard-to-heal leg ulcers: a randomised control trial. J Wound Care 2019; 28(10): 658-67. https://www. ncbi.nlm.nih.gov/pubmed/31600109
12. Tabanjeh SF, Al-Malki T, Kharabsheh RA, Mahmood D. A case series of autologous platelet-rich plasma injection in treating chronic ulcers conducted in Saudi Arabia. Int J Health Sci (Qassim) 2023; 17(2): 46-56. https://www.ncbi.nlm. nih.gov/pubmed/36891041
13. Clavel S, Denizot C, Boëzennec B, Turzi A, Albache NA. A randomized, controlled, clinical study comparing the efficacy of an autologous standardized leucocyte-poor platelet gel with standard care for the treatment of chronic neuropathic diabetic foot ulcers. Manuscript submitted for publication.
14. Russo S, Landi S, Courric S. Cost-Effectiveness Analysis for the Treatment of Diabetic Foot Ulcer in France: Platelet-Rich Plasma vs Standard of Care. Clinicoecon Outcomes Res 2022; 14: 1-10. https://www.ncbi.nlm.nih.gov/pubmed/35018103
15. Li S, Xing F, Yan T, Zhang S, Chen F. The Efficiency and Safety of Platelet-Rich Plasma Dressing in the Treatment of Chronic Wounds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Pers Med 2023; 13(3). https://www.ncbi.nlm.nih.gov/pubmed/36983611
16. Meznerics FA, Fehervari P, Dembrovszky F, Kovacs KD, Kemeny LV, Csupor D, et al. Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of clinical medicine 2022; 11(24). https://www.ncbi.nlm.nih.gov/pubmed/36556151
17. Peng Y, Wang J, Liu X, Zhou Y, Jia S, Xu J, et al. Efficacy of Platelet-Rich Plasma in the Treatment of Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Annals of vascular surgery 2024; 98: 365-73. https://www.ncbi.nlm.nih.gov/ pubmed/37355015
18. Platini H, Adammayanti KA, Maulana S, Putri PMK, Layuk WG, Lele J, et al. The Potential of Autologous Platelet-Rich Plasma Gel for Diabetic Foot Ulcer Care Among Older Adults: A Systematic Review and Meta-Analysis. Ther Clin Risk Manag 2024; 20: 21-37. https://www.ncbi.nlm.nih.gov/pubmed/38288358
19. Ruiz-Munoz M, Martinez-Barrios FJ, Fernandez-Torres R, Lopezosa-Reca E, Marchena-Rodriguez A. Autologous platelet- rich plasma (APRP) in diabetes foot disease: a meta-analysis. J Diabetes Complications 2024; 38(2): 108690. https:// www.ncbi.nlm.nih.gov/pubmed/38278034
20. Deng J, Yang M, Zhang X, Zhang H. Efficacy and safety of autologous platelet-rich plasma for diabetic foot ulcer healing: a systematic review and meta-analysis of randomized controlled trials. Journal of orthopaedic surgery and research 2023; 18(1): 370. https://www.ncbi.nlm.nih.gov/pubmed/37202812
21. Gong F, Zhang Y, Gao J, Li X, Zhang H, Ma G, et al. Effect of platelet-rich plasma vs standard management for the treatment of diabetic foot ulcer wounds: A meta-analysis. Int Wound J 2023; 20(1): 155-63. https://www.ncbi.nlm.nih. gov/pubmed/35751432
22. OuYang H, Tang Y, Yang F, Ren X, Yang J, Cao H, et al. Platelet-rich plasma for the treatment of diabetic foot ulcer: a systematic review. Front Endocrinol (Lausanne) 2023; 14: 1256081. https://www.ncbi.nlm.nih.gov/pubmed/38169990
23. Su YN, Li J, Feng DH, Lu RR, Dong GX, Zhao DY. Efficacy and safety of autologous platelet-rich plasma for diabetic foot ulcers: a systematic review and meta-analysis. J Wound Care 2023; 32(12): 773-86. https://www.ncbi.nlm.nih.gov/ pubmed/38060413
24. Hu Z, Wang S, Yang H, Xv H, Shan B, Lin L, et al. Efficacy and safety of platelet-rich plasma in the treatment of venous ulcers: A systematic review and meta-analysis of randomized controlled trials. Int Wound J 2024; 21(2): e14736. https:// www.ncbi.nlm.nih.gov/pubmed/38361238
25. Fang Q, Zhang Y, Tang L, Li X, Zhang X, Gang JJ, et al. Clinical Study of Platelet-Rich Plasma (PRP) for Lower Extremity Venous Ulcers: A Meta-Analysis and Systematic Review. The international journal of lower extremity wounds 2023; 22(4): 641-53. https://www.ncbi.nlm.nih.gov/pubmed/34665051
26. Yammine K, Ghanimeh J, Jil Agopian S, Assi C, Hayek F. PRP Versus Standard of Care for Venous leg Ulcers: A Systematic Review and Meta-Analysis of Prospective Comparative Studies. The international journal of lower extremity wounds 2022: 15347346221094424. https://www.ncbi.nlm.nih.gov/pubmed/35422142
27. Hu Z, Xv H, Feng A, Wang S, Han X. Efficacy and Safety of Platelet-Rich Plasma for Pressure Ulcers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. The international journal of lower extremity wounds 2024: 15347346241227001. https://www.ncbi.nlm.nih.gov/pubmed/38239009
28. Aleksandrowicz H, Owczarczyk-Saczonek A, Placek W. Venous Leg Ulcers: Advanced Therapies and New Technologies. Biomedicines 2021; 9(11). https://www.ncbi.nlm.nih.gov/pubmed/34829797
29. Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta- analysis.Eplasty2011;11:e38.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_ uids=22028946
30. Dai J, Jiang C, Sun Y, Chen H. Autologous platelet-rich plasma treatment for patients with diabetic foot ulcers: a meta- analysis of randomized studies. J Diabetes Complications 2020; 34(8): 107611. https://www.ncbi.nlm.nih.gov/ pubmed/32402839
31. Del Pino-Sedeno T, Trujillo-Martin MM, Andia I, Aragon-Sanchez J, Herrera-Ramos E, Iruzubieta Barragan FJ, et al. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis. Wound Repair Regen 2019; 27(2): 170-82. https://www.ncbi.nlm.nih.gov/pubmed/30575212
32. Ding H, Fu XL, Miao WW, Mao XC, Zhan MQ, Chen HL. Efficacy of Autologous Platelet-Rich Gel for Diabetic Foot Wound Healing: A Meta-Analysis of 15 Randomized Controlled Trials. Advances in wound care 2019; 8(5): 195-207. https:// www.ncbi.nlm.nih.gov/pubmed/31737414
33. Hirase T, Ruff E, Surani S, Ratnani I. Topical application of platelet-rich plasma for diabetic foot ulcers: A systematic review. World J Diabetes 2018; 9(10): 172-9. https://www.ncbi.nlm.nih.gov/pubmed/30364787
34. Hu Z, Qu S, Zhang J, Cao X, Wang P, Huang S, et al. Efficacy and Safety of Platelet-Rich Plasma for Patients with Diabetic Ulcers: A Systematic Review and Meta-analysis. Advances in wound care 2019; 8(7): 298-308. https://www.ncbi.nlm.nih. gov/pubmed/31832277
35. Li Y, Gao Y, Gao Y, Chen D, Wang C, Liu G, et al. Autologous platelet-rich gel treatment for diabetic chronic cutaneous ulcers: A meta-analysis of randomized controlled trials. J Diabetes 2019; 11(5): 359-69. https://www.ncbi.nlm.nih.gov/ pubmed/30182534
36. Putrantyo II, Mosahebi A, Smith O, De Vega B. Investigating Effectiveness of Topical Autologous Platelet-rich Plasma as Prophylaxis to Prevent Wound Infection: A Systematic Review and Meta-analysis. Malaysian Journal of Medicine & Health Sciences 2021; 17.
37. Qu W, Wang Z, Hunt C, Morrow AS, Urtecho M, Amin M, et al. The Effectiveness and Safety of Platelet-Rich Plasma for Chronic Wounds: A Systematic Review and Meta-analysis. Mayo Clin Proc 2021; 96(9): 2407-17. https://www.ncbi.nlm. nih.gov/pubmed/34226023
38. Qu S, Hu Z, Zhang Y, Wang P, Li S, Huang S, et al. Clinical Studies on Platelet-Rich Plasma Therapy for Chronic Cutaneous Ulcers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Advances in wound care 2022; 11(2): 56-69. https://www.ncbi.nlm.nih.gov/pubmed/33607926
39. Falanga V, Isseroff RR, Soulika AM, Romanelli M, Margolis D, Kapp S, et al. Chronic wounds. Nat Rev Dis Primers 2022; 8(1): 50. https://www.ncbi.nlm.nih.gov/pubmed/35864102
